Arlington, Virginia
February 10, 2005
The so-called Bt protein (Bt),
produced by the bacterium Bacillus thuringiensis, is
toxic to insects and widely used as an alternative to chemical
pesticides in organic farming and in other crops. Because the
mechanism the toxin uses to enter insect cells is not fully
understood, strategies to prevent insects from becoming
resistant to it are difficult to develop.
University of California San Diego
researcher Raffi Aroian and colleagues have discovered the first
step the toxin takes to enter the insect target cells. The
results of the work will be published in the Feb. 11 issue of
the journal Science.
Rita
Teutonico, program director in the eukaryotic genetics program
at the National Science Foundation, which supports the project,
said: “Dr. Aroian is uncovering the way pests become resistant
to Bt proteins. Understanding how resistance evolves
could alleviate concern about this natural pesticide losing its
effectiveness.”
The work also
confirms that the Bt protein is not toxic to vertebrates,
including animals and humans, since they lack the sugar
molecules to which the toxin binds.
As a bonus,
the Bt protein holds promise as a pesticide against
roundworms, because the worm’s sugar molecules are very similar
to those the toxin binds to in insects.
Related news
release from UC San Diego:
Discovery may
help extend life of natural pesticide
San Diego,
California
February 10, 2005
By Sherry
Seethaler
A team led by biologists at the University of California, San
Diego has discovered a molecule in roundworms that makes them
susceptible to Bacillus thuringiensis toxin, or Bt
toxin—a pesticide produced by bacteria and widely used by
organic farmers and in genetically engineered crops to ward off
insect pests.
Their
findings should facilitate the design and use of Bt
toxins to prevent insects, which the researchers believe also
possess the molecule, from developing resistance to Bt,
extending the life of this natural pesticide.
The study,
published February 11 in the journal Science, details the
structure of a molecule to which Bt attaches, or “binds,”
in the lining of the intestines of insects and roundworms. The
molecule is a glycolipid—a lipid attached to a tree-like
arrangement of sugars. Because changes in the sugars impact
Bt’s ability to bind, the researchers believe that their
discovery will make it possible to develop better pesticides and
lead to new treatments for parasitic infections that affect
close to two billion people worldwide.
|
 |
|
Image
of normal roundworm with graphic of Bt
toxin binding to molecule in intestine (top) and
resistant roundworm in which Bt cannot
bind.
Credit: Joel
Griffitts, Stanford University |
“Our previous
findings with the roundworm C. elegans strongly suggested
that specific sugar structures are likely critical for Bt
toxin susceptibility,” said Joel Griffitts, the first author on
the paper and a former graduate student with UCSD biology
professor Raffi Aroian. “This latest paper demonstrates what
these sugars actually do. They provide a receptor for the toxin
that allows the toxin to recognize its “victim”—a roundworm or
an insect. This paper also brings us from the conceptual realm
to the chemical nature of these sugar structures—how their atoms
are arranged, and how the toxin binds to them.”
“Bt
toxin, which is produced by a soil bacterium, is toxic to
insects and roundworms, but not to vertebrates, which accounts
for its popularity as a pesticide,” explained Aroian, who led
the team. “But the development of insect resistance to Bt
is a major threat to its long term use. Our findings make it
possible to understand resistance at the molecular level and
should improve resistance management.”
In
collaboration with Paul Cremer and Tinglu Yang, coauthors on the
paper and chemists at Texas A&M University, Griffitts and Aroian
found that Bt toxin directly binds glycolipids. However,
in each of the four Bt resistant mutants tested—bre-2,
bre-3, bre-4 and bre-5—the researchers found that
there was either zero or dramatically reduced binding of
glycolipids to Bt toxin. They concluded that the
defective sugar structure of the glycolipid receptor in each of
the mutants prevents Bt from binding.
Other members
of the research team, coauthors Stuart Haslam and Anne Dell,
biologists at Imperial College London; Barbara Mulloy, a
biochemist at the Laboratory for Molecular Structure, National
Institute for Biological Standards and Control in Hertfordshire,
England; and Howard Morris, a biochemist at the M-SCAN Mass
Spectrometry Research and Training Centre in Berkshire England,
determined the chemical structure of the normal glycolipid
receptor that binds Bt toxin.
Elements of
this structure are found in both insects and nematodes, but are
not found in vertebrates at all, which may be one reason these
proteins are safe to vertebrates. This work furthermore opens up
the possibility of using Bt toxins against roundworms
that parasitize humans.
“These
parasites infect nearly one-third of the human population and
pose a significant health problem in developing countries,” said
Aroian. “Perhaps one-day vertebrate-safe Bt toxins could
be used as human therapies against these parasites.”
Griffitts and
Aroian credit the flexibility of the roundworm C. elegans
as an experimental system, particularly the ease of manipulating
it genetically, in making it possible to find and characterize
the structure of the long sought-after Bt receptor.
However, their results apply to insects as well. Michael Adang
and Stephan Garczynski, coauthors and entomologists at the
University of Georgia, showed that the glycolipid receptor is
present in the tobacco hornworm, an insect pest that is
susceptible to Bt toxins used commercially in plants.
“It will now
be possible to monitor insect populations near fields where
Bt is used and catch insect resistance in its early stages
by looking for changes in glycolipids,” said Aroian. “If changes
are detected, switching to another pesticide, perhaps even
another variety of Bt that works through a different
mechanism, could prevent the resistance genes from becoming
widespread.”
According to
the researchers, prior work indicates that there are other
receptors that also contribute to Bt resistance.
Combining pesticides that work through different receptors or
designing pesticides that can work through more than one
receptor type could thwart the development of resistance.
“This paper
presents an intriguing question,” said Griffitts. “In light of
findings by insect biologists that certain proteins
function as important Bt toxin receptors in some cases,
how might glycolipid and protein receptors cooperate to engage
this intoxication program? If the field can figure this out, it
might allow for the engineering of toxins that can utilize
either type of receptor alternatively, such that host resistance
would require the mutation of both receptor types. This means
that resistance would be exponentially less probable.”
The study was
funded by the National Science Foundation, the
Burroughs-Wellcome Foundation and the Beckman Foundation.
Related news
release from UC San Diego:
San Diego,
California
August 2, 2004
UC San Diego
biologists identify genetic mechanisms conferring resistance to
Bt toxins
Biologists at
the University of California, San
Diego have discovered the genetic and molecular means by
which roundworms, and probably insects, can develop resistance
to the most widely used biologically produced
insecticide—crystalline toxins from the bacterium Bacillus
thuringiensis, or Bt.
Such Bt
toxins, which are safe to humans and other vertebrates and far
more environmentally friendly than pesticides, have been sprayed
on crops by organic farmers for decades. They have also played
an important role in
Africa in controlling insects that carry disease and are
now being used in genetically modified corn, cotton and other
crops to control caterpillars and beetles. But as the use of Bt
toxins expands worldwide, scientists fear their long-term
effectiveness will be threatened by the development of
Bt-resistant strains.
The
achievement by the UCSD biologists, reported in the August 3
issue of Science, provides important molecular and genetic
information that will help scientists develop strategies to
delay or circumvent the evolution of Bt-resistant strains of
roundworms and insects.
“There are
insects in the wild now that contain gene variants that allow
them to be resistant to Bt toxins, but fortunately they are
small in number,” says Raffi V. Aroian, an assistant professor
of biology at UCSD who headed the study. “However, as more crops
with Bt genes are planted, it’s only a matter of time before
populations of Bt-resistant insects grow numerous enough to
become economically troublesome to farmers hoping to control
these insects.”
In their
study, the researchers examined mutant genes they discovered in
the roundworm C. elegans that confer resistance to a particular
Bt toxin known as Cry5B. Joel S. Griffitts, a graduate student
at UCSD and the lead author of the study, cloned one of these
five mutant genes, which the scientists named bre for Bt
resistance, then compared differences in the proteins produced
by the mutant gene and the corresponding normal gene. That
comparison allowed the UCSD researchers, which included
postdoctoral fellow Johanna L. Whitacre and technician Daniel E.
Stevens, to conclude that the roundworm’s Bt toxin resistance
resulted from the loss of a galactosyltransferase, an enzyme
that adds carbohydrates to proteins and lipids.
Their
discovery prompted the scientists to hypothesize that
crystalline Bt toxins—which act by attacking and dissolving the
intestines of their hosts—normally recognize the outer surface
of intestinal cells by means of carbohydrates or sugars. When
the galactyosyltransferase gene is missing, these sugars are not
made and the toxin fails to recognize its host.
|
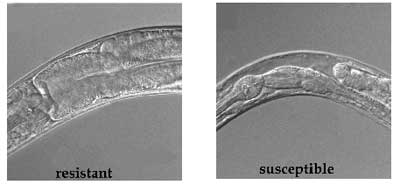
Resistant roundworms fed Bt toxin show no damage
to internal structures,
unlike the susceptible form. |
Whether this
enzyme is essential for many other Bt toxins remains to be
determined. But the UCSD scientists discovered that their mutant
roundworms were also resistant to a Bt toxin that is lethal to
beetles, suggesting that the development of resistance by the
loss of carbohydrate-modifying enzyme is relevant to insects as
well. Furthermore the three dimensional structure of diverse
insecticidal Bt toxins contains a fold that is predicted to bind
precisely the sugar modification made by the
galactosyltransferase, raising the possibility that this
mechanism of resistance could be widespread.
The discovery that the loss of a general modifier like a
galactosyltransferase can allow an organism to develop
resistance to Bt toxin is not good news.
“For people
using Bt toxins to control insects, this is a particularly
threatening scenario,” says Aroian. “The reason is that with one
swoop, you can knock out the binding of multiple toxins to
multiple receptors. But now that we know this mechanism of
resistance, we can devise strategies to cope with this.”
One possible
strategy, Aroian says, is for scientists to modify the toxins
such that they can bind to the inner lining of the insects’ or
roundworms’ guts independent of this carbohydrate modification.
In their
study, the UCSD scientists showed visually, using toxins labeled
with fluorescent dyes fed to normal and resistant forms of C.
elegans, that the Bt toxin is taken up into the gut cells of a
normal roundworm but not a resistant roundworm. If the toxin is
not recognized, as is the case in resistant animals, it simply
passes through the lumen of the gut and is defecated without
entering the gut cells.
|

In the wild-type, or normal, roundworms, the Bt
toxin (shown in red) readily moves into the gut
cells. In the resistant roundworms, the toxin
remains in the lumen and is soon eliminated. |
“This provides
strong evidence for our model, which essentially is that if you
don’t have this carbohydrate enzyme, you don’t make a
carbohydrate that the toxin needs to recognize the surface of
the gut,” says Aroian. “We also provide, using ‘mosaic
analysis,’ definitive molecular evidence that Bt toxins target
the gut. Scientists have long known that these toxins targeted
the gut. But this, at a molecular level, conclusively proves
it.”
The UCSD team’s discovery also sheds light on the puzzling and
sometimes contradictory findings of previous attempts to
pinpoint a mechanism for the development of Bt resistance in
insects.
“For a couple of decades now,” says Griffitts, the senior author
of the paper, “researchers have been grinding up insect guts and
finding components of those extracts that stick to Bt toxins.
And over the last decade, they’ve found multiple proteins, some
of which appear unrelated, that bind to Bt toxins. This study
may explain those seemingly contradictory results. These
proteins, which may look very different structurally, may have
the same binding motif because of carbohydrate modification.”
The discovery of this motif, or mechanism of action, in C.
elegans demonstrates the many advantages of this roundworm to
researchers. “The kind of analysis that can be done in C.
elegans can’t be done as easily in insects,” says Aroian. “We
have a complete genetic and physical map of C. elegans, we can
breed them in the laboratory easily, they grow fast, having only
a three-and-a-half day life cycle, they’re transparent, so we
can easily see their internal structures and they eat bacteria,
so we can express the Bt toxin right in their food source.”
The discovery of the resistance mechanism in C. elegans will not
only help farmers control insects. It will also help scientists
employ Bt toxins in the growing problem of roundworm, or
nematode, infestations in plants, animals and humans.
“Even if Bt toxins weren’t used to fight insects, nematodes are
a huge problem,” says Aroian. “At last estimate, which was 13
years ago, they caused $80-billion worth of crop damage per
year. And the damages will become worse, because the main
chemical now used to control them in agriculture, methyl
bromide, has been banned by the Montreal protocol. They are
also a human health problem—a quarter of the world’s population
are infected with animal parasitic nematodes.”
The UCSD study was supported by the National Science Foundation,
the Burroughs Wellcome Fund and the Beckman Foundation. |