Australia
August 3, 2005
 Paul
Grundy, Research entomologist, Qld DPI&F/ Cotton CRC explains
some of the new and innovative approaches to combating
insecticide resistance being trialled in Central Queensland. Paul
Grundy, Research entomologist, Qld DPI&F/ Cotton CRC explains
some of the new and innovative approaches to combating
insecticide resistance being trialled in Central Queensland.
Paul Grundy from
DPIF and the Cotton CRC has been doing work novel options in
resistance management for helicoverpa in Central Queensland
(CQ). Paul, what have you been doing and why?
What we have been looking at is
developing a more efficient Bollgard® resistance management
strategy for CQ. In particular what we have been focusing on in
this current season is looking at the whole trap cropping
program and ways that we could improve this strategy by using
products like Magnet®.
Why is there a need to improve on the
current system?
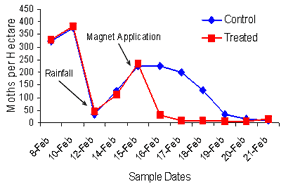 |
|
Moth flush counts |
Essentially the problem that we have
which is unique to CQ is that because our crop finishes that
little bit earlier, our conditions are warmer and the day
lengths aren’t receding quite as quickly as they do down south,
a large proportion of our heliothis would naturally escape those
fields before there is a chance to do pupae busting operations.
The system that we have had in CQ to
date is pigeon pea trap crops. The idea of that trap crop is
that the emerging moths that are escaping Bollgard® II paddocks
get attracted to the trap crops and we can then deal with them
mechanically by pupae busting them under these crops.
The problem that we have had with
trap crops in CQ though is that in some seasons our trap crops
don’t necessarily match up with when the emergence of the last
generation may occur. We have also had problems with the quality
of trap crops that have been grown. Pigeon pea can be quite
variable from season to season and with some of the water
restrictions that we have had, perhaps some of the pigeon pea
hasn’t been looked after quite as well as it could be.
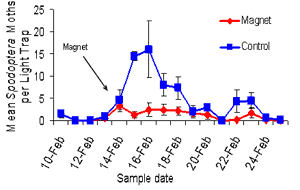 |
|
Light trap catches of
Spodoptera litura |
The other problem we have found with
trap crops is that they are an indirect way of targeting those
resistant or potentially resistant moths in that we are
capturing their progeny. What we thought with by looking at
Magnet® is that it is perhaps more direct in that we are
targeting; the moths themselves rather than targeting the eggs
and larvae from those moths.
How did you go
about measuring the effectiveness of the Magnet®?
What our question was for this season
is to see whether we could use the Magnet® on an area wide
scale. We went and picked our two areas in Theodore that were
roughly about 800 hectares in size each and we aerially applied
the Magnet® to one of those areas and then looked at doing moth
flush counts as well as light trap counts to see whether we
actually had an area wide impact on the moth numbers in the
treated areas as opposed to our control area.
Can you tell us
about those two methods of counting moths?
|
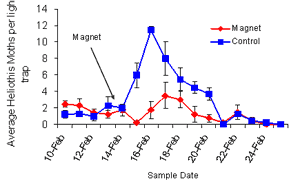 |
|
Light
trap catches of Helicoverpa |
Flush counts are a fairly basic
method of counting moths. What we do is walk into a crop, gather
a bucket full of soil out of that crop and we walk transects
through those crops where we throw the soil out in front of us
and count the moths as they emerge out of the crop.
This allows you to put a measure on
the moth numbers that are actually present and you can actually
work that out on a per hectare basis. This method gives us an
idea of the number of moths per hectare and we can get a
relative difference between the two treatments.
Using flush counts you can’t get a
specific idea of exactly what’s flying out in front of you so we
use our light traps to get down to a species level and tell
whether they are heliothis armigera, punctigera or some other
species.
So looking at the
two comparison areas, what did you find with your light trap
measurements and your flush measurements?
 |
Other pest species
Spodoptera litura most prevalent and observed as late
instars on BGII crops throughout the Dawson Valley
season long |
It was very interesting. When we put
the treatment on, we went with an aerial application late in the
afternoon and we found even by the following morning we had
about an 87% reduction in moth numbers in the treated area. We
went back on the second day and that reduction in numbers had
increased to about 97%. So, within about a 48hour period judging
from the flush counts we had quite considerable reductions. We
calculated out over that 800 hectares that we killed somewhere
around 400,000– 500,000 moths in that area over a week long
period. So it was quite a substantial result.
Part of the
implication of this work is resistance management for transgenic
crops. Can you ‘crystal ball’ a bit and tell me how this could
work?
What we are looking at is using
Magnet as a technology to replace trap cropping, targeting that
last heliothis generation that may be emerging from Bollgard®.
If we can bust those moths before they escape or as they start
escaping those fields and prevent them going off into the
surrounding environment, hopefully they are not going to come
back next year and cause us a problem. Really it’s about getting
a break in resistance and that’s how we are hoping this product
will fit a niche for our resistance management specific to CQ.
In Bollgard II®
there is what we are calling secondary pests coming through, has
this work showed up anything effective for any of the secondary
pests?
One of the pests that we noticed
within Bollgard® crops throughout the Dawson area this year in
particular was Spodoptera (cluster caterpillar). There were low
numbers of that pest throughout a lot of the Bollgard® II fields
and when we came to look at the actual moth numbers that we were
catching in our light traps we found that we had a significant
impact on spedoptra as a species as well. I think that is a real
fringe benefit with the approach of using Magnet® compared a
pigeon pea trap crop which is really only specific to heliothis,
so it’s a multiple approach affect.
Will this work
continue next season?
This season we have answered the
questions as to whether we can get area wide suppression with
Magnet® over a regional scale. What we really want to focus on
next season is replicating that result and coming up with a use
pattern strategy for it. For example, this season we went with a
1 in 72 row application. To be economically viable we really
need to start expanding that out to 1 row in 150 or 1 row in
200.
So it will be; how can we take this technology and make it fit
our system so that it allows us to meet our bottom line as well
as giving us our best possible chances for resistance
management?
WHERE TO FROM HERE?
Experiments this season will focus on identifying a probable
pattern that fits CQ scenario and building a statistically valid
measure of impact.
Acknowledgements:
CRDC
Ag Biotech
Moura Aerial Agriculture
Further Information:
Dr
Stephen Allen,
Robert Eveleigh, John
Marshall,
Craig
McDonald,
David
Kelly or
James
Quinn |