|
March, 2003
by Ray Wu and Ajay Garg
Department of Molecular Biology and Genetics
Cornell University, USA
ray.wu@cornell.edu
Rice is a major source of food for more than 2.7 billion people
on a daily basis. Rice is planted on about one-tenth of the
earth's arable land and is the single largest source of food
energy to half of humanity. Of the 130 million hectares of land
where rice is grown, about 30 percent contain levels of salt too
high to allow normal rice yield. Another 20 percent of this land
is periodically subject to drought conditions that routinely
affect food production. About 10 percent of the locations where
rice is grown occasionally experience temperatures that are too
low for healthy plant development. It is difficult to improve
rice tolerance against these abiotic stresses because they
involve not a single gene but a network of genes. Fortunately,
recent developments in transgenic approaches offer new
opportunities to elucidate the functions of many useful
candidate genes from different organisms and to improve the
resilience and yield of rice plants. Moreover, developing
salt-tolerant transgenic rice plants can introduce new areas of
land that currently contain salt too high to grow rice. It is
expected that genetically engineered, improved rice varieties
will help combat world hunger and poverty.
In general, plants respond to environmental stresses (drought,
excessive salinity, and low temperature) through a wide variety
of biochemical and physiological adaptive changes, such as the
accumulation of compatible solutes (glycine betaine, proline,
polyamines, and trehalose) and synthesis of many regulatory
proteins. One such compound is trehalose, a non-reducing
disaccharide of glucose, which plays an important role in stress
protection in a large variety of organisms ranging from bacteria
and fungi to invertebrate animals. For example, brine shrimp
eggs, commercially marketed as fish food or as pet "sea
monkeys," can remain dehydrated for years in a state of
suspended animation due to their trehalose content. Trehalose
also acts as a storage carbohydrate, and it possesses the unique
feature of reversible water absorption capacity to protect
biological structures from damage during desiccation. When water
dissipates from the shell of macromolecules (such as protein)
during severe dehydration, trehalose can act as a water
substitute on the surface of the dried protein.1 Thus, the
native folding and biological activity of proteins are
maintained, and denaturation and aggregation are prevented.
These protective properties of trehalose are clearly superior to
those of other sugars, such as sucrose, making trehalose an
ideal stress protectant.
Despite the wide distribution of trehalose in microorganisms and
invertebrates, trehalose had until recently only been found in a
few plant species, notably highly desiccation-tolerant,
resurrection plants [club mosses Selaginella lepidophylla and
the angiosperm Myrothamnus flabellifolius], so named because of
their unique ability to fully recover from a state of almost
complete loss of water. These resurrection plants can accumulate
trehalose at levels approaching 1% of dry weight under
non-stress
conditions, whereas the majority of plants do not appear to
accumulate easily-detectable amounts of trehalose. However,
genes that encode enzymes of trehalose synthesis, i.e.,
trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate
phosphatase (TPP) (Figure 1), have been recently identified in a
number of plants. This suggests that the ability to synthesize
low amounts of endogenous trehalose may be widely distributed in
the plant kingdom.
Recently, several research groups have attempted to study, via
genetic engineering, the role of trehalose in abiotic stress
protection and carbohydrate metabolism in plants. In all
previous studies, engineering constitutive overexpression of
TPS- and/or TPP-encoding genes from yeast or Escherichia coli in
tobacco or potato resulted in enhanced trehalose levels and
drought tolerance. However, constitutive overexpression of these
genes also leads to unfavorable
developments, such as stunted plant growth, lancelet leaves, and
altered roots, as well as changes in carbohydrate metabolism
under normal growth conditions.2,3,4,5
Recently, we reported an alternate strategy to engineer
increased trehalose accumulation in rice in such a way that
trehalose synthesis occurs only when there is abiotic stress. We
used a stress-inducible promoter to drive the overexpression of
Escherichia coli trehalose biosynthetic genes (otsA and otsB) as
a fusion gene (TPSP) for providing abiotic stress tolerance in
rice.6 The TPSP fusion gene7 has the dual advantages of
necessitating only a single transformation event to introduce
both genes simultaneously into the rice genome, while at the
same time increasing the catalytic efficiency for trehalose
formation by the bifunctional enzyme. |
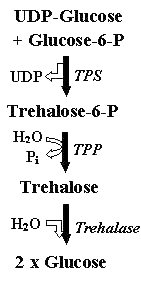 |
|
Figure 1.
Trehalose synthesis and degradation pathway in bacteria and
plants |
We introduced these two genes,
which are responsible for the synthesis of trehalose, into an important variety of rice plant (Pusa Basmati 1) by
Agrobacterium-mediated gene transfer and created a large number
of transgenic rice plants that are completely fertile and grow
well under normal growth conditions.(6)
The genetically-engineered rice plants produced higher amounts
of trehalose. Importantly, since our custom-designed inducible
promoter that drives the fusion gene is expressed only under
stress, the plants grow normally without any undesirable
effects. This is in contrast to previous experiments in which
researchers have constitutively expressed an individual TPS or
TPP gene so that it is turned on all the time, which stunts the
growth of plants.
Furthermore, the transgenic rice plants exhibited sustained
plant growth, less photo-oxidative damage, and more favorable
mineral balance under salt, drought, and low-temperature stress
conditions as compared to non-transgenic plants, many of which
died due to salt stress. Depending on growth conditions, the
transgenic rice plants accumulate trehalose at levels 3 to 10
times that of the non-transgenic controls. The observation that
peak trehalose levels remain well below 1 mg/g fresh leaf or
root weight indicates that the primary effect of trehalose is
not just serving as a compatible solute. Rather, increased
trehalose accumulation correlates with higher soluble
carbohydrate levels and an elevated capacity for photosynthesis
under both stress and non-stress conditions, consistent with a
suggested role in modulating sugar sensing and carbohydrate
metabolism. These findings demonstrate the feasibility of
engineering rice for increased tolerance of abiotic stress and
enhanced productivity through stress-dependent or
tissue-specific overproduction of trehalose.6
Our laboratory has experimented with six other genes, each of
which provides some degree of stress tolerance. What is special
about the two genes responsible for the overproduction of
trehalose is that the degree of protection from stresses has
been much higher than with the other genes reported previously.
In conclusion, we have demonstrated that engineering trehalose
overproduction in rice can be achieved by stress-inducible or
tissue-specific expression of a bifunctional TPSP fusion gene
without any detrimental effect on plant growth or grain yield.
During abiotic stress, transgenic plants accumulated increased
amounts of trehalose and showed high levels of tolerance to
salt, drought, and low-temperature stresses, as compared to
non-transgenic plants. These results demonstrate the potential
use of our transgenic approach in developing new rice cultivars
with increased abiotic stress tolerance and enhanced rice
productivity. In principle, this same technique can be used to
confer stress tolerance on other high-value, sensitive crops
such as wheat and corn.
References
1. Crowe JH, Hoekstra FA, Crowe LM. 1992. Anhydrobiosis. Annu
Rev Physiol 54:579-599.
2. Goddijn OJ et al. 1997. Inhibition of trehalase activity
enhances trehalose accumulation in transgenic plants. Plant
Physiol 113:181-190.
3. Holmstrom KO et al. 1996. Drought tolerance in tobacco.
Nature 379:683-684.
4. Pilon-Smits EAH et al. 1998. Trehalose-producing transgenic
tobacco plants show improved growth performance under drought
stress. J Plant Physiol 152: 525-532.
5. Romero C et al. 1997. Expression of the yeast
trehalose-6-phosphate synthase gene in transgenic tobacco
plants: pleiotropic phenotypes include drought tolerance. Planta
201: 293-297.
6. Garg AK et al. 2002. Trehalose accumulation in rice plants
confers high tolerance levels to different abiotic stresses.
Proc Natl Acad Sci USA 99: 15898-15903.
7. Seo HS et al. 2000. Characterization of a bifunctional enzyme
fusion of trehalose-6-phosphate synthetase and
trehalose-6-phosphate phosphatase of Escherichia coli. Appl
Environ Microbiol 66: 2484-2490.
|